
Consider that as an SOP list that you can start to see if this is applicable to your business.
QUALITY MANUAL ISO 13485 2016 PDF ISO
It’s your ISO 13485 documentation to build your own Quality System by yourself.
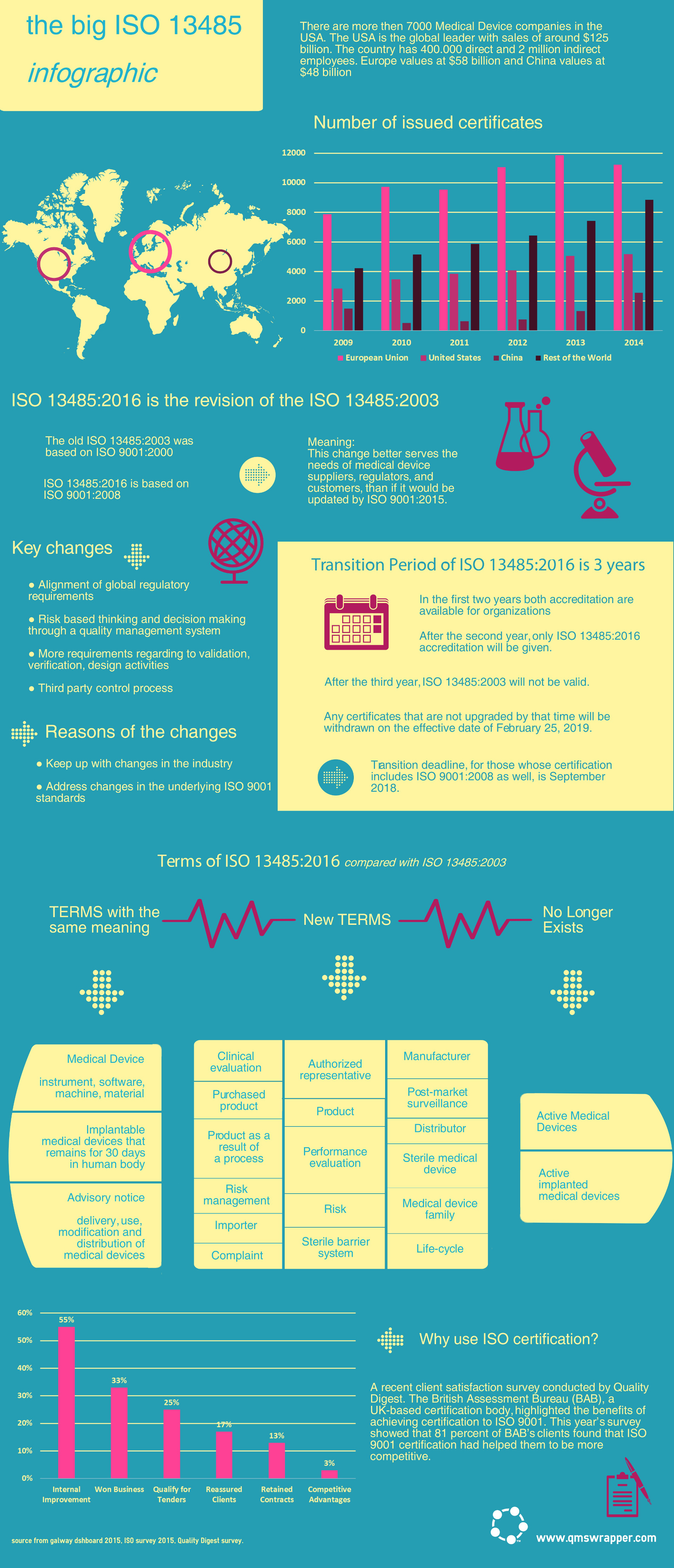
Their applicability will be dependent on the specific products and regulatory requirements of the countries and regions where products are distributed. It will help them quickly understand the purpose of audits conducted against the standard and enable them to better fulfill their roles with clarity and confidence.įor orders of 25 or more books we can customize the front and back covers of this pocket guide to suit your company logo, colors, and message to employees. I listed on an excel sheet the documents that are mentioned on the ISO 13485:2016 standard. Quality Manual, their listing as references does not imply compliance with all of them. This pocket guide will assist each employee in answering the multitude of questions that occur while implementing and maintaining a compliant medical devices/services quality system in the workplace. Quality Management System requirements impact every employee. Title: Quality Policy Manual ISO 9001:205:2016 AS9100D:2016 Document: Q1 Revision: P5 Date: Protomatic Quality Manual Q1 Rev P5 APPROVED. Key points of the requirements for each clause are highlighted in yellow, while blue highlighting is used to quickly locate the likely actions of an auditor. – Enables employees to better perform their duties with more confidence and less concernĮach book contains a front end Q & A, a discussion of quality, and explains the value of being a registered company. – Captures the most likely actions of the auditor in each clause The quality management system was established to meet the requirements of ISO 9001:2015 Quality Management Systems-Requirements, our customers, interested parties and the organization. – An easy reference and a quick reminder as to what to expect in an audit – Describes in detail the role and expectations for management and employees in each clause The committee responsible for this document is Technical Committee ISO/TC 210, Quality management and corresponding general aspects for medical devices. – Explains the meaning and purpose of each clause of the standard ISO 13485 is a stand-alone standard, therefore has got similarities with ISO 9001 Quality Management System in the scope and intent. – Pocket size and spiral bound for ease of use The third column shows if the new medical device regulation could have some additional impact on these processes and procedures or not.The ISO 13485:2016 Pocket Guide for Every Employee (2nd Edition) is your quick reference and helpful guide to implementing and maintaining the ISO 13485 quality standard. The second column lists all of the affected chapters of ISO 13485:2016 for additional information. The list below represents the main processes and procedures required by the ISO 13485:2016. It is thus the basis for companies that are in the field of medical technology. ISO 13485:2016 can also be used by suppliers or external parties that provide products, such as quality management system-related services to such organizations.

Such organizations can be involved in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities.

ISO 13485:2016 specifies requirements for a quality management system of an organization that needs to demonstrate its ability to provide medical devices and related services and consistently meet customer and applicable regulatory requirements.


 0 kommentar(er)
0 kommentar(er)
